Multivalent VLPs are effectively produced using HEK 293T cells
To determine the optimal incorporation of proteins onto VLPs, we transfected human embryonic kidney cells (HEK 293T cells) with different ratios of expression plasmid DNA for NiV M, F, G (Malaysia strain), HeV F, G (original Australian strain), and EBOV GP (Zaire strain), for a total of 30 µg of plasmid DNA per 15-cm dish (Fig. 2A). Incorporation of proteins onto the VLPs was confirmed using standard Western blot analysis. From the initial results of experiments such as that shown in Fig. 2A, we expanded the total µg of DNA used to 45 µg/15-cm dish and tried various DNA ratios (Fig. 2B). Transfection of cells with the DNA ratio 7:12:12:2:2:10 µg for NiV M, NiV F, HeV F, NiV G, HeV G, and EBOV GP, respectively, yielded the best protein incorporation into virions (Fig. 2B) and expression of the proteins in cell lysates (Fig. 2C), determined by fluorescent Western blot analysis quantification. Individual protein blots with molecular weight markers for VLPs are shown in supplementary Fig. 11. We also determined the cell surface expression (CSE) levels for each glycoprotein by flow cytometry (Fig. 2D). Incorporation of the proteins onto VLPs was determined by flow virometry34 (Fig. 2E). The results demonstrated that multiple glycoproteins can be found on the surfaces of viral particles of a single viral preparation. We demonstrated the presence and distribution of the glycoproteins on the surfaces of VLPs by electron microscopy. Figure 2F shows a TEM micrograph of VLPs and their spikes, which were not observed in negative control bald particle samples. These figures demonstrate that different immunogenic glycoproteins can be incorporated onto VLPs following transfection of HEK 293T cells.
Fig. 2: Optimized production of NiV-HeV-EBOV VLPs and their protein detection using multiple techniques.
A Different amounts of DNA (µg) were used to transfect HEK 293T cells to determine the closest range for each target protein for optimized production detected by Western Blot analysis (WBA). B Shows the best VLP production DNA ratio detected by WBA. C WBA detection of proteins in cell lysate of cells transfected to produce VLPs in (B). D The cell surface expression (CSE) of the target proteins analyzed by Flow Cytometry (FC). E Detection of the target proteins on VLPs using Flow Virometry (FV). F A micrograph of the produced VLPs viewed using a FEI T20 electron microscope.
Multivalent pseudotyped VSV particles are effectively produced using HEK 293T cells
Similarly, as in Fig. 2, we transfected HEK 293T cells with varying amounts of expression plasmid DNA for NiV F/G, HeV F/G, and EBOV GP for 12 h and then infected them with VSV-rLuc virions as was applied in previous studies and depicted in Fig. 335,36.
Fig. 3: Pseudotyped virion production system.
A diagram showing transfection of HEK 293T cells using plasmids carrying DNA encoding for the immunogenic proteins of interest, followed by infection using VSV-deltaG virus. The subsequent pseudotyped VSV virion preparation carries all five distinct viral glycoproteins and can be used as a multivalent vaccine.
From the initial Western blot analysis (Fig. 4A), we decided that the best pseudotyped VSV protein incorporation would be achieved after transfection with DNA amounts of about 5 to 7 µg for each protein expression plasmid. We therefore transfected the HEK 293T cells with 6 µg DNA to express each of the NiV, HeV, and EBOV surface glycoproteins (Fig. 4B) for incorporation onto the pseudotyped VSV. The proteins were also detected in the cell lysates by Western blot analysis (Fig. 4C). The Western blots with molecular markers for the detection of Pseudotyped VSV particles and lysates are shown in supplementary Fig. 11. The cells used to produce pseudotyped VSV particles were stained in a similar manner to those used for VLP production. The levels of glycoprotein incorporation were determined by flow virometry (Fig. 4D), and expression of the glycoproteins on the pseudotyped VSV was also determined by flow cytometry (Fig. 4E). Multiple glycoproteins were found on individual viral particles. Figure 4F shows a TEM micrograph of a pseudotyped VSV particle showing surface glycoproteins, again, not observed in negative control samples. We also demonstrated the presence of more than one type of glycoprotein on the surface of individual virus particles by flow virometry (Fig. 4G, H). Figure 4G shows an example of the gating of individual viral particles containing both NiV F and HeV G on single particles. The same gating strategy was used to determine the percentage of double-positive virions for various other pairs of glycoproteins that contained extracellular (extraviral) tags (Fig. 4H).
Fig. 4: Optimized production of Pseudotyped VSV incorporating NiV-HeV-EBOV glycoproteins.
A Different amounts of DNA (µg) were used to give the closest range for each target protein for optimized production detected by WBA. B WBA detection of proteins in cell lysate of cells transfected to produce the pseudotyped VSV particles. C Shows the best DNA ratio for production of pseudotyped VSV detected by WBA. D The cell surface expression (CSE) of the target proteins analyzed by FC. E Detection of the target proteins on pseudotyped VSV using FV. F A micrograph of the produced pseudotyped VSV particles viewed using a FEI T20 electron microscope. G Flow cytometry data showing an example of the presence of double-positive viral populations (red) as compared to the negative control bald particles (blue). H Percentage of viral populations double positive for various pairs of glycoproteins with extracellular tags. One representative experiment of three is shown.
Trehalose lyophilization improves viral particle thermostability
To determine the thermostability of the pseudotyped virions, we used the cell entry capabilities of the monovalent and multivalent VSV virions as a surrogate of particle stability. Their luciferase marker gene allowed us to assess viral particle entry. We made pseudotyped VSV virions incorporating NiV G only (negative control for viral entry), NiV F/G, HeV F/G, EBOV GP only, or all NiV F/G, HeV F/G and EBOV GP (multivalent virions). Bald virions with plasmids pCDNA3.1/pCAGGS served as an additional negative control for viral entry. The pseudotyped virions were used to infect Vero cells at a confluency of 30% and VSV luminescence was recorded 24 h post-infection (hpi). Figure 5A shows the cell entry levels of the monovalent and the multivalent virions.
Fig. 5: Carbohydrates preserve the thermostability of pseudotyped VSV particles.
A Vero cell entry capabilities for pseudotyped VSV incorporating monovalent and multivalent target proteins determined using Renilla Luciferase assay. The relative MOI of the pseudotyped VSV was determined based on luminescence produced from the Luc gene and read as relative light units (RLU). The pseudotyped VSV virions were collected 40 h post transfection. The monovalent and multivalent pseudotyped VSV virions were diluted 1:100 to 1:1,000,000 and their entry capabilities determined to assess the optimal entry levels for the monovalent and multivalent pseudotyped VSV. B Determination of Vero cell entry of non-lyophilized multivalent pseudotyped VSV exposed to 4 °C, 25 °C, and 37 °C for 42 days. C Determination of sucrose and trehalose capability to preserve the multivalent pseudotyped VSV during lyophilization. D Determination of viability for the 5% w/v trehalose preserved pseudotyped VSV. Viability was determined using the Renilla Luciferase assay.
After determining the entry capabilities of the pseudotyped virions, we then determined their stability under different temperature environments. We exposed the multivalent virions to temperatures of 4 °C, 25 °C, and 37 °C for a period of up to six weeks and determined their entry levels into Vero cells, as in Fig. 5A. The virions were stable at 4 °C and 25 °C for the six weeks but lost a log in luminescence detection within a week when exposed to 37 °C, and luminescence detection was in the range of the negative controls by the end of the second week (Fig. 5B).
Thermotolerance of virus vaccines has been enhanced using carbohydrates37. We compared the capacity of sucrose and trehalose to preserve the multivalent pseudotyped virions following a lyophilization process. The virions were lyophilized in 2.5% or 5% sucrose or trehalose for 24 h at 1:100 and 1:1000 dilutions. Following lyophilization, 5% trehalose preserved the pseudotyped virions completely, yielding luciferase levels similar to those of fresh non-lyophilized virions (Fig. 5C). We then lyophilized the multivalent pseudotyped virions using 5% trehalose and exposed them to temperatures of 4 °C, 25 °C, and 37 °C. Positive control lyophilized virions were preserved under vacuum at 4 °C for the six-week period, while we placed vials of virions under three different temperatures starting with the day 42 vials, and ending with the day 0 vials (we placed the day 42 vials first, then day 35 vials after a week, then days 28, 21, 14, and 7 in that order), so that all vials were tested on the same day (day 0). The Day 0 lyophilized sample (Fig. 5D) represents values for vials at kept at 4 °C for the entire period. There was minimal loss in viability of the pseudotyped virions following lyophilization when stored at 4 °C or 25 °C, as measured by viral entry levels. Remarkably, we observed a minimal loss in luminescence for the lyophilized virions for up to five weeks at 37 °C, a high environmental temperature. Therefore, lyophilization in 5% trehalose improved thermotolerance of the pseudotyped VSV virions, allowing the use of this type of vaccine in high-temperature climates where maintenance of a cold-chain is impractical.
The multivalent VLPs elicited neutralizing antibody responses in hamsters
Following vaccination, we determined the immunogenic properties of the VLPs in hamsters. We vaccinated five hamsters intramuscularly at six weeks of age using 50 µl (30 µg) of the vaccine preparation in 50 µl of ALUM adjuvant and boosted on days 21 and 42 post first inoculation. Five negative control hamsters were vaccinated with 50 µl of bald VLP preparation in 50 µl of ALUM adjuvant and boosted on days 21 and 42 after first inoculation. The amount of protein inoculated was determined by Bradford assay. Blood samples were collected weekly from day 0 to day 49. Final bleed serum neutralization was determined against fresh batches of pseudotyped VSV virions incorporating NiV F/G only, HeV F/G only, EBOV GP only, or a multivalent VSV virions carrying the three viral glycoproteins using a Renilla Luciferase kit 24 h.p.i.38. Supplementary Figs. SF1A to SF1E show graphs of monovalent and multivalent pseudotyped VSV neutralized with different dilutions of antisera from individual negative control hamsters, showing no antibodies against the NiV F/G, HeV F/G, or EBOV GP. Supplementary Figs. SF1F to SF1J show graphs of monovalent and multivalent pseudotyped VSV virions neutralized with different dilutions of sera from individual VLP-vaccinated hamsters. Figures SF1K to SF1O are sigmoidal graphs derived from neutralization read outs for the sera from the individual VLP-vaccinated hamsters. Figure 6A shows the averaged neutralization for all negative control hamsters, while Fig. 6B shows the averaged neutralization trends for sera from hamsters vaccinated with VLPs. Figure 6C is a sigmoidal curve derived from the average neutralization data for sera from hamsters vaccinated with VLPs. The VLP-vaccinated hamsters elicited neutralizing antibodies against all NiV F/G, HeV F/G, and EBOV GP, but were weakest against EBOV.
Fig. 6: Multivalent VLPs and pseudotyped VSV incorporating NiV F/G, HeV F/G, and EBOV GP elicited neutralizing antibodies in hamsters.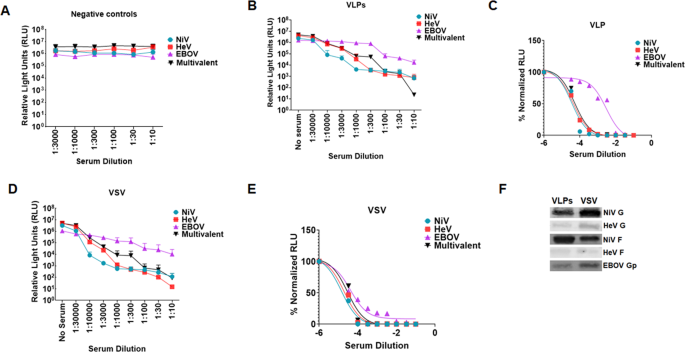
A. Negative control hamsters were vaccinated with bald VLPs. Serum for the hamsters’ terminal bleed was used to neutralize monovalent NiV F/G, HeV F/G, EBOV GP, and the multivalent pseudotyped VSV particles and entry of the virus into the cells was analyzed by Renilla Luciferase assay. The mean neutralization read out for the monovalent and multivalent pseudotyped VSV was calculated and graph plotted using Graphpad software. B Monovalent and multivalent pseudotyped VSV were neutralized with different dilutions of sera from hamsters vaccinated with multivalent VLP vaccine. Virus neutralization was done in a similar manner as the negative controls. The average neuralization read outs were calculated and graph derived using Prism Graphpad software. C Hamsters were vaccinated with the multivalent pseudotyped VSV. Sample processing was done as for the negative controls. The mean neutralization read out was calculated and graph derived using Prism graphpad software. D, E Normalized graphs for sera from hamsters vaccinated with the multivalent VLPs and pseudotyped VSV. F Forty microliters of VLP and pseudotyped VSV preparations were analyzed by Western blotting to compare incorporation of glycoproteins following observation that pseudotyped VSV was eliciting a relatively stronger immune response compared to VLPs.
The multivalent pseudotyped VSV virions elicited strong neutralizing antibody responses in hamsters
We also immunized different groups of hamsters with the pseudotyped VSV virions. These groups were treated similarly to the group which was immunized with VLPs. Serum samples were also treated as those collected from hamsters vaccinated with the VLPs. Figures SF2A to SF2E show graphs of monovalent and multivalent pseudotyped VSV neutralized with different dilutions of sera from the multivalent pseudotyped VSV vaccinated hamsters. Figures SF2F to SF2J show sigmoidal graphs derived from neutralization for sera from the pseudotyped VSV vaccinated hamsters. Figure 6D shows the averaged neutralization graphs for antisera derived from hamsters vaccinated with the multivalent pseudotyped VSV vaccine. Figure 6E is a sigmoidal curve derived from the average neutralization read outs for hamsters vaccinated with pseudotyped VSV. The pseudotyped VSV vaccinated hamsters elicited strong neutralizing antibody responses against all NiV F/G, HeV F/G, and EBOV GP. Comparison of Fig. 6C to E illustrates that the neutralizing antibody responses to the multivalent VSV vaccine were stronger than those to the multivalent VLP vaccine, likely due to the higher level of incorporation of the glycoproteins into the VSV virion vaccine per equal amount of sample tested, coincidently produced roughly from equal amounts of cells (Fig. 6F). Because the multivalent pseudotyped VSV preparation had higher incorporation of the glycoproteins (except for NiV F) and it elicited a stronger antibody response, it was subsequently used for the challenge experiments. We also quantified the amount of IgG in the sera for the mock vaccinated hamsters and that from VLPs and pseudotyped VSV vaccinated hamsters using an ELISA. The data shows detection of similar levels of IgG antibodies among all groups indicating that increased neutralization was not due to an increase in total antibodies produced, but to specific neutralizing antibodies (SF 3).
Multivalent vaccines elicited time-dependent neutralizing antibody responses
After determining the terminal-bleed neutralizing antibody responses to the VLPs and pseudotyped VSV vaccines, we determined the weekly responses starting from vaccination priming, as performed for the terminal bleed samples. Figure 7 shows the averaged results of monovalent pseudotyped VSV for NiV, HeV, and EBOV VSV virions neutralized using the weekly bled sera. The results indicate that immune responses to NiV and HeV develop very early after vaccination priming, while that for EBOV lags behind but becomes stronger a week after vaccination boosting (i.e., day 28). Generally, as expected, neutralizing antibody titers continued to improve thereafter for the testing period.
Fig. 7: Multivalent pseudotyped VSV and VLPs elicited time-dependent neutralizing antibody titers.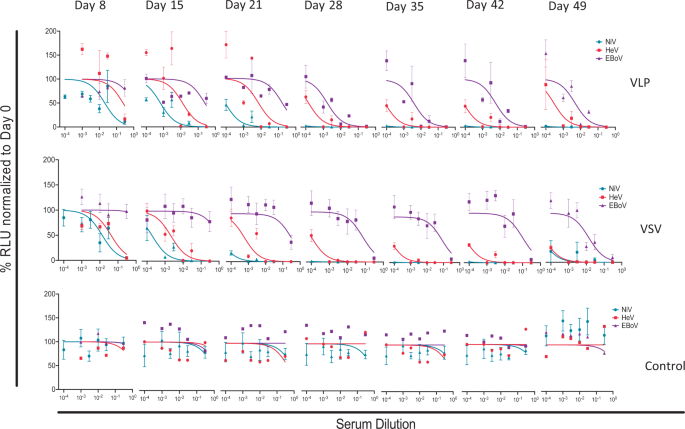
Neutralization tests were performed for individual hamsters, and the results shown are averages of individual hamster sera. Sera from pseudotyped VSV and VLP-vaccinated hamsters and control groups were analyzed for eight time points and data normalized to day 0. Error bars represent standard deviation.
Multivalent pseudotyped VSV protected hamsters against NiV, HeV or EBOV challenge
We then vaccinated a new batch of hamsters as performed for Fig. 6. The hamsters were grouped into six groups of six hamsters per group. The first eighteen hamsters were mock vaccinated with phosphate-buffered saline (PBS) and Alum, and the second group of eighteen hamsters vaccinated with the multivalent pseudotyped VSV test vaccine (Fig. 8). Sera from the vaccinated hamsters was tested for neutralizing antibodies against NiV, HeV, and EBOV prior to challenge as performed in Fig. 6. The hamsters vaccinated using the multivalent pseudotyped VSV vaccine elicited a strong antibody response (SF 4A, B, and C). Further, sera from these hamsters also neutralized live NiV, HeV, and EBOV (SF 4C, D, and E). In the mock and test vaccine groups, six hamsters were separately challenged with NiV, HeV, or mouse adapted EBOV (maEBOV) five days after arrival at the NIAID Integrated Research BSL-4 laboratory (Fig. 8).
Fig. 8: The study design.
The figure indicates the vaccination schedule for all hamsters used in this study, the amounts of mock and test vaccines applied, the point of challenge, and the period that the challenge experiment lasted.
All hamsters in the test vaccine group survived challenge with the three viruses and exhibited no adverse signs of disease (Fig. 8A), although we lost one maEBOV-challenged hamster to an unrelated lymphoma. All mock vaccinated hamsters had to be euthanized due to severe disease post-challenge (Fig. 9A). Hamsters challenged with NiV had to be euthanized by day 9, those challenged with HeV by day 5, and those challenged with maEBOV by day 8 post-infection (Fig. 9A). There was also a decrease in body weight and temperature for the mock vaccinated group during the course of the experiment as shown in Fig. 9B, C, respectively. During the experiments, the hamsters were also scored clinically based on appearance, respiration, mobility, body temperature, neurological signs, paralysis, seizures, and moribund status. The highest score was 15, which was attained if a hamster was unable to access food or water, had a 4 °C drop in body temperature from the baseline and if it was moribund. Other scores fell between 0 and 15. Hamsters were monitored daily during early, mid, and late hours. All the hamsters vaccinated with the multivalent VSV vaccine did not show any deviation on any of the parameters from the baseline, while all the mock vaccinated hamsters recorded a significant deviation on a number of these parameters prior to euthanasia (Fig. 9D). In summary, these challenge experiments demonstrated that the test multivalent VSV vaccine protected hamsters from challenge from virulent NiV, HeV, and EBOV infections at 100% efficacy and with 100% safety, ~4 months post-vaccination.
Fig. 9: Analysis of hamster survival, weight, and temperatures during the course of challenge experiments.
A Percent survival of test-vaccinated vs. mock-vaccinated hamsters, following challenge with live NiV, HeV, or maEBOV. Hamsters vaccinated with the test vaccine survived challenge with all the three virulent viruses except that one hamster died from a lymphoma (unrelated cause). Those vaccinated with mock VSV virions were euthanized at critical control points after signs of disease. B Weight records for the test vs. mock vaccinated hamsters groups. Hamsters in the test vaccine group showed minimal variation on weight during the course of experiments while those in the mock-vaccinated groups registered significant weight loss prior to euthanasia. C Body temperature records for the mock vs. test-vaccinated groups. Hamsters test-vaccinated showed minimal variation in body temperatures while the variation was marked for the mock groups. D Clinical scores for the multivalent pseudotyped VSV vaccinated hamsters compared the mock-treated group. Clinical scores were based on a 15-point scale that includes scoring appearance, respiratory signs, mobility, temperature, and neurological signs such as paralysis, seizures, and appearing moribund. Additive scores of 15 or greater required immediate consultation with the veterinarian to determine animal disposition. There was marked deviation of clinical scores between test and mock vaccinated groups. Solid lines represent test-vaccinated animals, while dashed lines represent mock-vaccinated animals.
Multivalent pseudotyped VSV vaccinated hamsters did not suffer pathology following challenge with virulent NiV, HeV, and EBOV
Following euthanasia, different tissues were collected from the challenge hamsters for histopathology. Tissues used for analysis included the brain, liver, and lung. Figure 10A, C demonstrates presence of pathology in the brain and lung, respectively, for mock-vaccinated and NiV challenged hamsters, but not for the multivalent pseudotyped VSV vaccinated and NiV challenged hamsters (Fig. 10B, D). Pathology and results due to NiV challenge were similar to those caused by HeV challenge. Figure 10E, G demonstrates pathology in the liver and lung, respectively, in the mock vaccinated and EBOV challenged hamsters. The multivalent pseudotyped VSV vaccinated and EBOV challenged hamsters did not present pathology (Fig. 10F, H).
Fig. 10: Multivalent VSV virion vaccination prevented the development of histologic lesions in hamsters challenged with Nipah, Hendra, or Ebola viruses.
Tissues from representative mock vaccinated (A, C, E, G) or multivalent VSV virion vaccination (B, D, F, H) following challenge with either Nipah (A–D) or Ebola virus (E–H). A Brain tissue from a representative mock vaccinated hamster with inflammatory perivascular cuffs (arrows). C Lung tissue from a representative mock vaccinated hamster with intravascular fibrin thrombi (arrowhead), edema in alveoli (asterisk), and type II pneumocyte hyperplasia with atypia (arrow). E Liver tissue from a from a representative mock vaccinated hamster with lobular hepatitis, hepatocyte necrosis (arrow), and intracytoplasmic viral inclusions (arrowhead). G Lung tissue from a representative mock vaccinated hamster with intravascular fibrinocellular debris (arrows) and expansion of alveolar septa with inflammatory cells (arrowhead). Lesions caused by Hendra virus resembled those caused by Nipah virus. H&E, scale bar = 50 μm.
Sera from the multivalent pseudotyped VSV vaccinated hamsters cross-neutralized pseudotyped VSV incorporating glycoproteins for Cedar virus
Given the successful neutralization of both NiV and HeV, we then asked whether a related henipavirus, Cedar virus (CedV), with moderate sequence similarities to NiV and HeV G and F (SF 5A) may also be neutralizable with the vaccinated sera. We first tested the binding levels of sera from the vaccinated multivalent VSV hamsters on HEK293T cells expressing the surface G or F glycoproteins from either NiV or CedV. Interestingly, we observed that the sera bound relatively well particularly to the F proteins (SF 5B). Since F is relatively more conserved than G (SF 4A), this raises the possibility of serum cross-neutralization of other henipaviruses. We thus tested one serum with particularly good F binding levels (serum 6.2) for neutralization of pseudotyped CedV. At a 1:30 dilution of this serum, we observed neutralization of pseudotyped CedV (SF 5C). Altogether, these results suggest that the multivalent vaccination strategy is capable of yielding cross-protective antibodies against henipaviruses beyond NiV and HeV, justifying further use and optimization of this vaccination strategy towards the goal of broadly protective vaccines.
CD4+ T cell depletion during vaccination prevents CD4+ and CD8+ cell restimulation to vaccine antigens
To assess whether T cells respond to viral antigens after vaccination, we harvested splenocytes from CD4+ T cell-depleted or isotype antibody control treated hamsters 3 weeks after delivery of multivalent VSV intramuscular vaccinations (SF 6). Notably, while CD8+ T cell depletion was not effective in hamsters using available anti-CD8 antibodies, circulating CD4+ T cells were depleted to less than 1% at the time of vaccination, and slowly rebounded to an average of ~35% of control animal CD4+ T cell counts by day 20 (SF7). Splenocytes were stained with dye that can report on proliferation, cultured for 5 days in the presence of media alone, pseudotyped bald virus control (PBC), monovalent NiV F/G (NiV), HeV F/G (HeV), EBoV GP (EBoV), or multivalent pseudotyped viruses, or with ConA stimulation as a positive control (SF 8). Cells were then harvested and stained for flow cytometry analysis to determine proliferated cells (gating strategy in SF 8). Proliferation of CD4+ (SF 9A) and CD8+ (SF 9B) T cells were compared between the isotype control and the CD4+ T cell-depleted hamsters. CD4+ and CD8+ T cells of both groups showed robust capacity for proliferation following stimulation with the ConA as a positive control (expected since that by day 20, CD4+ T cell counts in the CD4+ T cell depleted animals had rebounded to an average ~35% of control animals). Due to a variation in cell toxicity of each pseudotyped virus, we could not compare across pseudotyped virus treatments, only between isotype and CD4+ T cell-depleted hamster groups within each activation treatment. Interestingly, all pseudotyped viruses induced proliferation of both CD4+ and CD8+ T cells from the isotype antibody control treated hamsters. However, in almost all cases, cells from the CD4+ T cell-depleted hamsters were unable to proliferate in response to the pseudotyped viruses. These data indicate that some form of T cell immune memory is generated by immunization with the multivalent VSV pseudotyped vaccines, and that the presence of CD4+ T cells at the time of vaccination is necessary for adequate vaccine antigen restimulation of CD4+ and CD8 T+ cells three weeks later.
CD4+ T cell depletion did not significantly change neutralizing antibody production
CD4+ cells were depleted from hamsters39 with an intraperitoneal antibody injection 24 h prior to vaccination intramuscularly with multivalent VSV pseudotyped viruses. Blood was collected weekly post vaccination, and serum from the isotype control or CD4+ T cell-depleted hamsters was evaluated for ability to neutralize monovalent NiV F/G, HeV F/G, or EBoV GP pseudotyped viral entry into cells. Viral entry was quantified by measuring Renilla Luciferase activity, normalized to levels from pre-vaccination serum. Despite slight trends of decreased neutralization activity from the CD4+ T cell-depleted hamsters, there was no statistically significant difference in neutralizing antibody titers between the two groups (SF 10).
Source: news.google.com